Prof. HUANG Qing’s group from the Institute of Intelligent Machines, Hefei Institutes of Physical Science (HFIPS), has developed a surface-enhanced Raman spectroscopy (SERS) gas sensor to detect aldehyde with high sensitivity and selectivity. The sensor has provided a new detection method for studying the adsorption of gas molecules on porous materials.
Adsorption technology is one of the main technologies for treating Volatile organic compounds (VOCs). Metal-organic frameworks (MOFs) have attracted high interest for their outstanding adsorption property. Closely related to MOFs, layered double hydroxides (LDHs), also known as hydrotalcite-like systems or anionic clays, have been noted for their improved adsorption properties due to the enhanced porosity and chemical affinity at multiple active sites.
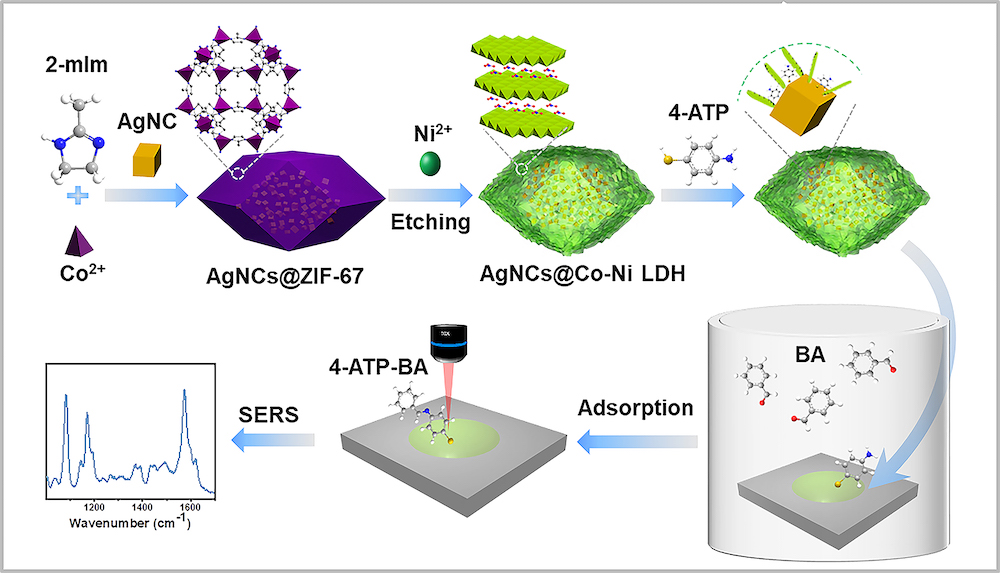
In this study, silver nanocubes (AgNCs) and Co-Ni LDH composite nanomaterials were prepared in template sacrifice method, and modified with 4-aminophenol (4-ATP) for both trapping and probe functions. Based on the as-prepared composite material, researchers constructed a high-efficiency gas sensor for selective detection of aldehyde gas.
“This SERS sensor has ultrahigh sensitivity for aldehyde gas,” said XU Di, the first author of this paper. “We verified its accuracy, repeatability and selectivity in the experiment.”
Combined with principal component analysis method, the team successfully identified and analyzed the similar SERS spectrum of aldehyde gases with the sensor, indicating application value.
They further investigated the adsorption kinetics and thermodynamic process of benzaldehyde molecules on Co-Ni LDH with the sensor.
The kinetic adsorption process could be fitted better by the pseudo-first-order kinetics with a higher correlation coefficient than by a pseudo-second-order model.
The isotherm adsorption fits the Langmuir isotherm model, and its adsorption constant is 6.25 × 106 L/mol, indicating that the adsorption sites of the composites were homogeneous and dominated by monolayer chemisorption.
This study established a new measurement method for probing the adsorption process with extremely low consumption of both adsorbates and adsorbents, but also may lay the groundwork for the construction of rapid and ultra-sensitive SERS sensors for probing VOCs in the future.